An article written by Dr Edward Leatham, Consultant Cardiologist © 2025 E.Leatham
Tags: VAT, Metabolic Health, NH1, search website using Tags to find related stories.
In a related article, I covered our philosophy for diagnosis and early detection of cardiovascular and metabolic diseases.
Detecting risk is only the first step. At SCVC, we offer a progressive, tiered framework of interventions that can be scaled up or down, depending on patient response and risk levels.
Self-toolkit: daily feedback and behavioural modulation
We provide tools that patients can use individually or in combination, to adjust lifestyle and diet and gradually improve insulin dynamics and reduce VAT.
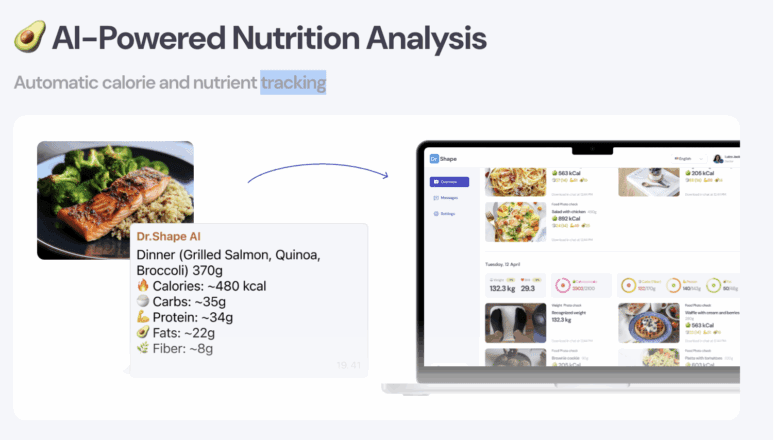
- DoctorShape food analysis app (via WhatsApp)The DoctorShape app is deployed over a three-month contract. Through WhatsApp, patients receive daily caloric and macronutrient goals. They input their meals in real time (via messaging, photos or text), and receive immediate feedback against their prescribed profiles. This tool helps patients steer their dietary composition, often guiding toward increased protein, moderated carbohydrate intake and better macronutrient balance.

Many patients find this real-time feedback extremely useful: it keeps them engaged, responsive, and aware of their intake decisions, rather than relying solely on retrospective logs.
Home exercise and muscle-strengthening routines
Patients receive guided daily or near-daily exercise plans (as little as 10 minutes a day) using bodyweight or dumbbell routines and video instruction. The goal is to build skeletal muscle mass, increase mitochondrial density, and boost insulin sensitivity—all powerful levers against metabolic syndrome and VAT.

Body composition scales and smart tape measures
To provide objective feedback, patients are supplied or encouraged to use smart scales and tape measures that estimate skeletal muscle mass, total body fat, and VAT (or proxies). Tracking changes over days to weeks helps maintain motivation and fine-tune coaching.


Nurse-led professional programme (6 months)

For patients preferring more structured guidance, we offer a six-month nurse-led VAT-reduction programme (excluding pharmacotherapy). Under the supervision of a specialist diabetic nurse, patients receive telephonic and face-to-face support, dietary coaching, exercise supervision, and group interaction (via the DoctorShape WhatsApp group). This structured oversight improves adherence and outcomes.
Escalation: GLP-1 microdosing or full therapy under oversight
If after six months of structured intervention the patient is insufficiently responsive (in terms of VAT reduction, glucose control, or risk metrics), we provide an option to escalate therapy with GLP-1 receptor agonist injections over 6 months. In some cases patients have already exhausted their efforts to manage their excessive VAT/ weight and need help from the start- so can be referred by heir clinician to our 8-month metabolic reset programme.
This is a hybrid programme combining lifestyle, digital support, and pharmacotherapy. Patients may begin with microdosing GLP-1, or full-dose therapy, depending on baseline risk, tolerability, and metabolic phenotype. All medication is prescribed by our consultant cardiologists or endocrinologists; dosing and monitoring is overseen by the specialist nurse. The programme includes helpline support, integration with the DoctorShape app and periodic reviews.The primary objective is to reduce VAT, improve insulin sensitivity, and shift metabolic trajectory. Importantly, many patients who respond well are able to taper off GLP-1 therapy after the reset period, maintaining improvements via lifestyle alone.
This tiered model—from pure lifestyle tools, to nurse-led coaching, to pharmacotherapy if required—ensures that therapy is personalised, efficient, and not over-medicalised when not needed.
Imaging and phenotypic feedback to close the loop
We emphasise feedback physiology: nothing drives adherence more than seeing objective improvement. To this end we integrate imaging and other phenotypic metrics:
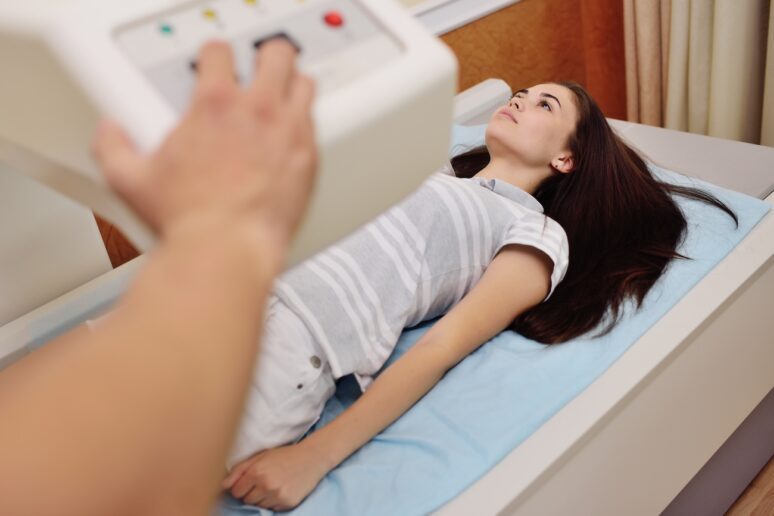
DEXA scanning
To quantify skeletal muscle, total body fat, bone density and lean mass, DEXA scans are periodically deployed. These give high-precision body composition snapshots and help detect subtle shifts not captured by weight alone.

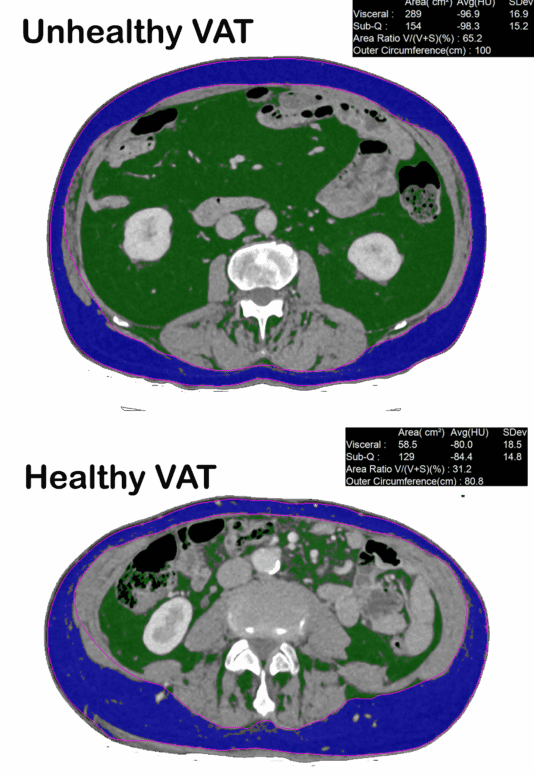
Low-dose CT imaging (1 mm non-contrast slice)
Using carefully optimised protocols (around one quarter of a mammogram dose), we measure the visceral adipose tissue (VAT) cross-sectional area. This CT slice gives a “ground truth” quantification of VAT burden, which we correlate with metabolic risk and guide patient progress.By pairing VAT imaging with FAI and plaque imaging, we can relate metabolic intervention directly to vascular inflammation and plaque dynamics.

Thus, the combined arsenal—CGM, composition scans, CT VAT, FAI imaging—allows us to monitor responses in glucose physiology, adiposity, and vascular health in parallel.
Who benefits? Patient streams and clinical indications
The cardiometabolic services at SCVC are highly relevant for a variety of patient groups:
- Asymptomatic individuals with risk factors
Patients with family history of coronary disease, borderline hypertension, dyslipidaemia, central obesity or insulin resistance can benefit from early phenotyping and targeted intervention far before symptoms or events occur. - Patients with early coronary disease or non-obstructive plaque
In those who already have evidence of coronary plaque or raised FAI, metabolic intervention becomes even more critical. Lowering VAT, smoothing glucose, reducing inflammation and optimising lipid therapy can slow or reverse plaque progression. - Hypertension, atrial fibrillation or heart failure risk
Many patients with hypertension or early atrial fibrillation have a metabolic substrate—abundant VAT, insulin resistance, chronic inflammation—that contributes to disease burden. In these cases, VAT reduction may be a more physiological route than increasing pharmacotherapy. - Patients with evidence of metabolic dysfunction not meeting diabetes criteria
Those in the so-called non-diabetic hyperglycaemia spectrum (e.g. frequent glycaemic spikes, insulin resistance) may not meet diagnostic cutoffs, but they remain at high risk of progression and cardiovascular injury. Intervening early is likely more effective than delaying until overt diabetes.
Because our programmes offer multiple entry points (self, nurse, pharmacotherapy), patients can choose the level of engagement that suits them, while always retaining the option to escalate as needed.
Philosophy and patient experience
From the outset, our philosophy is to treat patients as partners in their metabolic journey, not passive recipients. Key guiding principles include:
- Phenotype before protocol
Each patient’s metabolic profile is unique. We do not deliver one-size-fits-all diets or drugs; we begin with phenotype (CGM profiles, lipid panels, imaging) and tailor dietary, exercise and pharmacologic prescription accordingly. - Continuous feedback and adaptation
Real-time tools (CGM, app-based food feedback) plus periodic imaging allow us to detect plateaus or suboptimal responses quickly, and adjust strategies iteratively. - Tiered escalation, not overmedicalisation
We aim first to optimise lifestyle and behavioural interventions. Only when these are insufficient do we escalate pharmacotherapy (GLP-1). Even then, our philosophy is that reducing pharmacotherapy where possible is better than indefinite therapy. - Holistic behavioural support
Metabolic disease is deeply entwined with psychology, habits, stress, sleep, and environmental factors. We integrate behavioural coaching, psychological support and motivational frameworks into our programmes. - Transparency and patient empowerment
Patients receive reports, progress graphs, and coaching rationale. The more they understand their own physiology, the more engaged and committed they become.
From onboarding through to follow-ups, patients typically comment that the service feels deeply personalised, dynamic, and informed by data—not generic.
Illustrative case (anonymised, from clinic practice)
To bring this to life: one 64-year-old male patient presented for cardiometabolic screening. He had a prior transient ischaemic attack, elevated LDL cholesterol treated with statin therapy, and a strong family history of myocardial infarction, but was a non-smoker, with normal blood pressure and HbA1c in the “normal” range. However, on CaRi-Heart imaging, his CT showed extensive non-obstructive plaque and elevated FAI across all three coronary vessels, placing him in a high-risk category. (Surrey Cardiovascular Clinic)
Meanwhile, his CGM revealed multiple glycaemic excursions exceeding 7.8 and 10 mmol/L, starkly divergent from what one would expect in a “normal-HbA1c” individual. (Surrey Cardiovascular Clinic)
The intervention plan included:
- Escalation of lipid-lowering therapy, with aggressive LDL target (e.g. <1.6 mmol/L)
- Dietary modification guided by CGM feedback: elimination of high glycaemic foods (e.g. honey in porridge, excessive fructose-laden fruit)
- Use of CGM biofeedback to titrate and refine diet
- Optimization of his antihypertensive therapy (adjusted via ambulatory BP monitoring)
- Engagement with our structured behavioural and nutritional coaching
Over the ensuing months, the patient reported progressive reductions in waist circumference, smoother glucose profiles, and improved imaging metrics. The integrated approach (imaging + CGM + dietary change + therapeutic intensification) provided both motivation and measurable validation of progress. To review details of imaging, CGM and CaR heart see related article
This case encapsulates our philosophy: early detection of disease activity, metabolic phenotyping beyond conventional labs, personalised intervention, and iterative feedback to close the loop.
The scale and impact of the service
While the ambition of SCVC’s cardiometabolic services is significant, the scale is accessible. Many elements (CGM use, app feedback, home exercise) are feasible for remote or geographically dispersed patients, especially those willing to engage digitally. The imaging and nurse-led programmes provide additional scaffolding for higher-risk individuals.
By embedding metabolic optimisation within cardiovascular prevention, we are helping shift the paradigm from late-stage rescue interventions to early, individualized, physiology-guided prevention. For cardiologists, endocrinologists and general physicians, this model offers several key advantages:
- Patients with hypertension or atrial fibrillation who carry significant metabolic burden may derive more long-term benefit from VAT reduction and insulin sensitivity improvement than escalation of drug therapy alone
- Early pharmacologic intervention (e.g. GLP-1) can be more effective and safer when administered in the context of lifestyle support, phenotypic feedback, and imaging endpoints
- The alignment of vascular imaging (FAI) with metabolic change offers a unique opportunity to observe whether metabolic interventions truly “move the needle” on vascular health
- The tiered model ensures cost efficiency—only those needing drug therapy escalate, while others may succeed on behavioural programmes alone
From a public health perspective, the ability to identify patients decades before overt disease—and to intervene meaningfully—could reduce long-term rates of myocardial infarction, heart failure, and related morbidity.
Challenges, limitations and areas for development
As with any pioneering model, SCVC’s cardiometabolic services confront challenges:
- Adherence and behavioural inertia
Lifestyle change remains hard. Even with feedback tools and coaching, many patients plateau or revert. We mitigate this with psychological support, motivational frameworks, and frequent feedback loops—but the human factor remains crucial. - Cost, access and scalability
Imaging, CGM, nurse support and app infrastructure incur costs. Ensuring affordability and access for a broader population (beyond those paying privately) remains a key consideration. - Interpreting phenotypic heterogeneity
Not all patients respond equally. Some may have underlying genetic predispositions or less modifiable drivers. Tailoring further in such cases is complex. - Long-term constraints of pharmacotherapy
Even though many patients may be able to withdraw GLP-1 after the reset period, some may require maintenance therapy. Optimising tapering strategies and avoiding rebound becomes an area for further study. - Validation of endpoints
While FAI and VAT imaging offer promising biomarkers, long-term prospective validation is still evolving. Demonstrating that metabolic interventions reduce hard endpoints (MI, stroke, death) will require long-term follow-up.
Nonetheless, the current early experience suggests that many patients make steady, clinically meaningful progress with the integrated model.
Looking forward: the future of cardiometabolic care at SCVC
The vision is expansive. Over the coming years, we aim to:
- Expand remote access (telemedicine, remote CGM, app-based coaching) to regional or national patient cohorts
- Integrate more advanced biomarkers (e.g. metabolomics, proteomics, genetic risk scores) into phenotyping
- Leverage artificial intelligence to personalise intervention pathways dynamically
- Conduct longitudinal outcome studies to correlate metabolic change with event reduction
- Partner with primary care, endocrinology, and public health systems to disseminate the model
In serving patients with hypertension, coronary disease, atrial fibrillation or isolated metabolic dysregulation, we see tremendous opportunity. The arsenal of tools—imaging, CGM, app coaching, nurse-led pathways and GLP-1 therapy—is sufficiently broad to fit most patient phenotypes. By making prevention more precise, dynamic and patient-centred, we hope SCVC will serve as a model for future cardiometabolic centres across the UK.
Conclusion: an invitation to join the metabolic paradigm shift
We live in a time when cardiovascular and metabolic diseases present as a convergent epidemic. A patient with early coronary atherosclerosis is often not just a vascular case—but a metabolic one. By targeting visceral adiposity and glucose dysregulation early, we have the chance to change trajectories, not just manage late-stage disease.
At Surrey Cardiovascular Clinic we have built a full stack of cardiometabolic tools—imaging, metabolic phenotyping, coaching, behavioural support, escalation paths, and iterative feedback—that works together as a system. For patients with risk, for clinicians seeking more effective prevention, and for a health system fighting the burden of cardiovascular disease, the potential impact is substantial.
If you’d like to explore a patient pathway, refer a case, or collaborate further in cardiometabolic research or service development, I’d be delighted to discuss. Together we can help more people shift from risk to resilience.
Related posts
- Cardiometabolic toolkit
- Visceral Fat, Mitochondria, and the Energy Trap: Why We Store Fat Where We Shouldn’t
- Carbohydrate Sensitive Phenotype (CSP): Precursor of the Metabolic Syndrome?
- Exercise and Digital Tools Should Be the First Line in Reducing Visceral Fat in Cardiac Patients
- Cardiologists and a New Enemy: Evolving Tools of the Trade
- Carbohydrate Sensitive Phenotype (CSP): Precursor of the Metabolic Syndrome?
- Metabolic Health Assessment
- Biofeedback: CGM metrics improve after just 4 weeks of dietary intervention
- Lets compare continuous glucose monitor (CGM) results