An article written by Dr Edward Leatham, Consultant Cardiologist © 2025 E.Leatham
Tags: VAT, Metabolic Health, NH1, search website using Tags to find related stories.
For busy people, or to tune in when on the move, Google Notebook AI audio podcast and an explainer slide show are available for this story beneath.
You’ve probably heard mitochondria described as the powerhouses of the cell. That’s true — but it’s just the beginning.
Mitochondria do not just keep your cells alive — they help determine how your body handles fuel, how you age, and how vulnerable you are to inflammation and chronic disease.
These microscopic organelles sit at the crossroads of energy, metabolism, and immunity. In this article, we explore what mitochondria are, how they work, and what happens when modern life — and modern diets — overwhelm them.
🧬 A Legacy from Your Mother — and from Bacteria
Mitochondria are unique: they are the only part of your cells (other than the nucleus) that carry their own DNA. In fact, they were once free-living bacteria that entered into a partnership with early human cells more than a billion years ago.¹
All your mitochondria come from your mother, passed through the egg at conception. Over time, these tiny symbionts became indispensable — managing your energy, responding to demand, and protecting vital organs like the brain, heart, and muscles.
⚙️ What Mitochondria Actually Do?

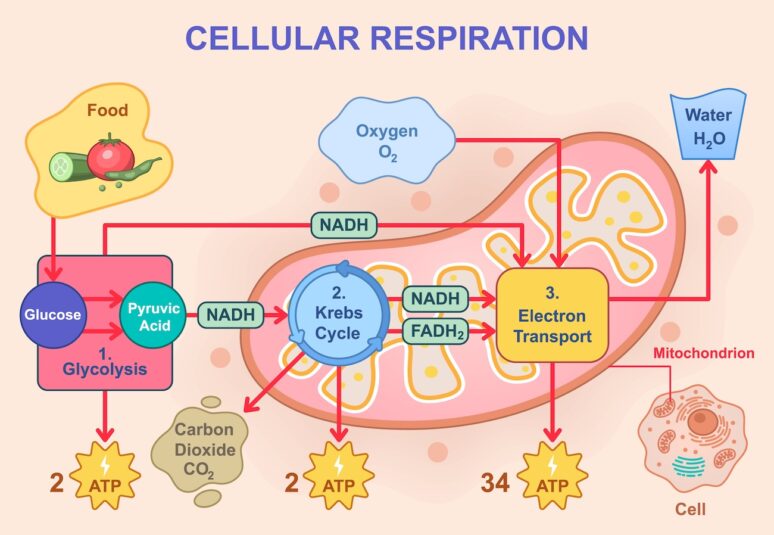
Vector illustration of cellular respiration showing glycolysis, Krebs cycle, and electron transport chain. Glucose breakdown, ATP production, oxygen intake, and water and carbon dioxide release
Mitochondria produce ATP (adenosine triphosphate) — the energy currency of life. They do this via oxidative phosphorylation, a process that extracts electrons from glucose and fats to power a proton gradient and generate ATP.When running efficiently, this system powers everything from heartbeats to hormone synthesis to brain activity. But mitochondria do not have a storage tank — they can only produce ATP on demand.
Which brings us to their biggest vulnerability: energy surplus
🌞 The Solar Panel Analogy: What Happens in Energy Overload

Energy storage power station where solar generation surplus (glucose) to grid (body) needs will be stored in battery banks (adipose).
Think of mitochondria like solar panels. On a sunny day, they convert sunlight (glucose) into electricity (ATP). But what if the batteries are full, and you keep producing more electricity?
There is no off switch.
The excess electricity has nowhere to go, so it starts to overload the system. Wires heat up. Fuses blow. Small fires start. The infrastructure, once efficient, is now damaged.
This is exactly what happens in our cells when:
- Fuel intake (especially glucose) is chronically high
- Energy demand (movement, repair, adaptation) is low
- Storage systems (like glycogen and adipose tissue) become saturated
The mitochondria become overwhelmed. Electrons leak from the electron transport chain and react with oxygen to form free radicals (ROS) — sparking damage and inflammation.³
🛡️ Insulin: The Body’s Internal Circuit Breaker
Under normal conditions, the body protects mitochondria from overload by releasing insulin. This hormone acts as a safety valve, diverting excess glucose into storage:
- Some is stored as glycogen in muscle and liver
- The rest is converted into triglycerides and stored in adipose tissue
It is a beautiful, homeostatic design — one that evolved to support a hunter-gatherer life, where food was irregular, and long periods without calories were common. The system was built to manage feast and famine, not three carb-heavy meals plus snacks every day.
🚨 When Storage Overflows: The Problem with Modern Fuel Supply
Today, many people live in a constant “solar surplus” as they age — high-calorie diets, sedentary lifestyles, skeletal muscle loss (sarcopenia), and minimal variation in intake.
Subcutaneous and then Visceral fat (VAT) starts to expand — the latter not just as storage, but then it evolves into an inflammatory organ in its own right⁵ .At first, insulin copes. It tries to pack glucose into muscles, then liver, then visceral fat cells. But over time:
- Muscles become less responsive to insulin (insulin resistance), made worse by VAT release of free fatty acids and cytokines
- Fat cells become inflamed and dysfunctional
- The liver fat storage is overloaded
At this point, insulin’s protective role starts to fail. Glucose levels rise in the bloodstream despite insulin release after carbohydrate intake and floods mitochondria, which can no longer regulate the surplus. This phenomenon seems to occur in some people with an innate predisposition more than others – a term dubbed carbohydrate sensitive phenotype (CSP), a prodrome to non diabetic hyperglycaemia, pre-diabetes and type 2 diabetes.
That is when the real damage starts to nibble away at your healthspan without clear symptoms or visible signs, except perhaps some aches and pains, an expanding collar and waist dimensions.
- Free radical production (ROS) increases dramatically
- Oxidative stress damages membranes and DNA
- Immune cells are activated, triggering inflammation
- High insulin levels also contribute to arterial cellular inflammation
🔥 Mitochondria and Inflammation
When overloaded mitochondria produce ROS and leak mitochondrial DNA, this activates innate immune responses, including the NLRP3 inflammasome, which triggers inflammatory cytokines like IL-1β.⁶
These signals are meant to protect — but in chronic energy surplus, they remain activated, leading to metabolic inflammation.
This “invisible fire” contributes to:
- Fatigue
- Brain fog
- Insulin resistance
- Atherosclerosis
- Ageing-related tissue damage (‘inflammation’)
👵 Ageing and Mitochondrial Decline
As we age, mitochondrial function naturally declines:
- Mitochondria accumulate DNA mutations
- Their ability to divide and regenerate falls
- Oxidative damage increases
- The body becomes less efficient at removing damaged mitochondria (via mitophagy)
The result? Reduced energy, poor glucose handling, and greater vulnerability to disease.
This decline is accelerated by chronic mitochondrial overload — exactly what is caused by constant glucose excess and sedentary living.⁷
💡 How to Protect and Reboot Mitochondria
The good news? Mitochondria are highly responsive to lifestyle change. Here is how to keep them strong:
1. Exercise
- Increases mitochondrial number (biogenesis)
- Improves glucose disposal
- Supports mitochondrial repair and turnover⁸
2. Time-Restricted Eating / Fasting
- Triggers mitophagy (clearing out damaged mitochondria)
- Reduces ROS and inflammation
- Resets mitochondrial gene expression⁹
3. Lower Glycaemic Load
- Avoid glucose spikes that overwhelm the system
- Use CGM to guide food choices and eating patterns, especially if you have CSP.
4. Prioritise Protein and Muscle Mass
- Muscle is a major site of mitochondrial activity and glucose clearance
- Preventing sarcopenia reduces metabolic stress in later life
5. Micronutrients and Antioxidants
- Magnesium, B vitamins, CoQ10, omega-3s all support mitochondrial enzymes
- Polyphenols from olive oil, green tea, berries reduce oxidative stress
🧠 Final Thoughts
Mitochondria are at the centre of how we handle energy. They keep us alive, alert, and adaptable — until they are overwhelmed.
Modern life asks our mitochondria to do something they were never designed for: process surplus glucose every few hours, day after day, without movement, fasting, or rest.
That is why mitochondrial stress is now recognised as a driver of inflammation, insulin resistance, and ageing.
In a related post, we will look more closely at what happens when energy that cannot be burned or stored begins to accumulate as visceral fat — and how this “invisible organ” becomes both a symptom and a cause of metabolic disease.
References
- Gray MW. Mitochondrial evolution. Cold Spring Harb Perspect Biol. 2012;4(9):a011403.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3428776/ - Giles RE et al. Maternal inheritance of human mitochondrial DNA. PNAS. 1980;77(11):6715–9.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC35056/ - Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417(1):1–13.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2605959/ - Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in insulin resistance. Nature. 2006;440(7086):944–8.
https://doi.org/10.1038/nature04634 - Wensveen FM et al. The “Big Bang” in obese fat: events initiating obesity-induced adipose tissue inflammation. Eur J Immunol. 2015;45(9):2446–56.
https://pubmed.ncbi.nlm.nih.gov/26220361/ - Shimada K et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome. Immunity. 2012;36(3):401–14.
https://doi.org/10.1016/j.immuni.2012.01.009 - López-Otín C et al. The hallmarks of aging. Cell. 2013;153(6):1194–217.
https://doi.org/10.1016/j.cell.2013.05.039 - Lanza IR et al. Exercise as a means to improve mitochondrial function in aging. J Clin Invest. 2015;125(2):615–23.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4319423/ - Madeo F et al. Caloric restriction mimetics: towards a molecular definition. Nat Rev Drug Discov. 2014;13(10):727–40.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4295154/
Related posts
- Visceral Fat, Mitochondria, and the Energy Trap: Why We Store Fat Where We Shouldn’t
- Carbohydrate Sensitive Phenotype (CSP): Precursor of the Metabolic Syndrome?
- Exercise and Digital Tools Should Be the First Line in Reducing Visceral Fat in Cardiac Patients
- Cardiologists and a New Enemy: Evolving Tools of the Trade
The Naked Heart is an educational project owned and operated by Dr Edward Leatham. It comprises a series of blog articles, videos and reels distributed on Tiktok, Youtube and Instagram aimed to help educate both patients and healthcare professionals about cardiology related issues.
If you would like to receive email notification each week from the Naked Heart, follow me on social media or please feel free to subscribe to the Naked Heart email notifications here