An article written by Dr Edward Leatham, Consultant Cardiologist © 2025 E.Leatham
Tags: VAT, GLP-1, Inflammation, Diet, Arthritis, search website using Tags to find related stories.
For busy people, or to tune in when on the move, Google Notebook AI audio podcast and an explainer slide show are available for this story beneath.
Joint pain—hips, knees, back, fingers, hands—is a common complaint in overweight and obese individuals. Traditionally, improvements in joint symptoms following weight loss have been attributed to reduced mechanical load: less weight equates to less strain on load-bearing joints. But increasingly, both clinical observations and scientific evidence suggest another mechanism may be at play.
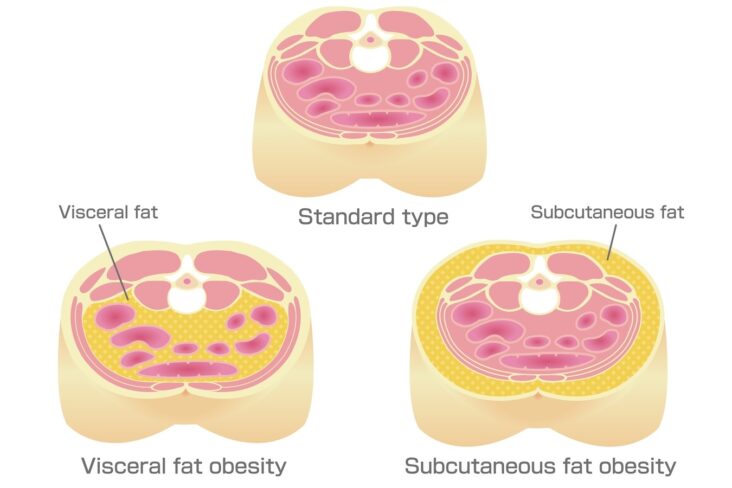
Adipose tissue—particularly visceral adipose tissue (VAT)—is not merely a passive energy store. It is metabolically active and pro-inflammatory, secreting cytokines and adipokines that drive systemic inflammation. It is therefore plausible that reducing VAT may alleviate joint pain by lowering inflammatory signalling, not just mechanical stress.
In this article, we explore the hypothesis that weight loss reduces arthritis and joint pain by lowering inflammatory mediator release from VAT, rather than load-bearing mechanics alone.
Visceral Adipose Tissue: An Inflammatory Organ
Not all fat is created equal
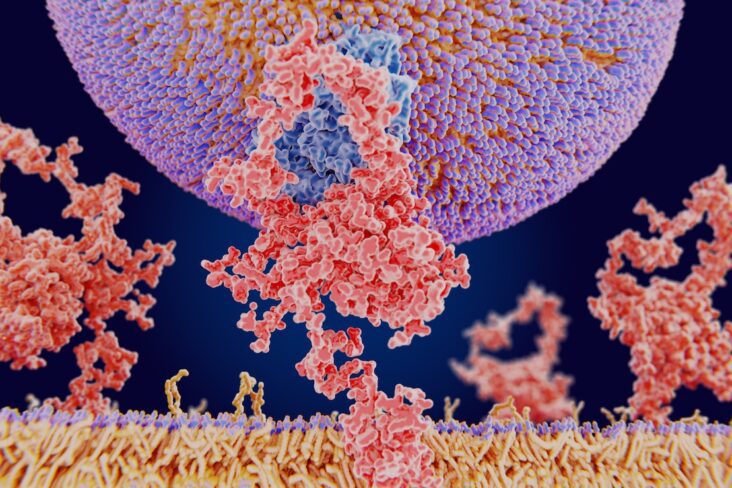
VAT is biologically distinct from subcutaneous fat. It is more vascularised, more innervated, more densely infiltrated with immune cells (especially macrophages), and more prone to hypoxia and fibrosis. Importantly, VAT secretes a range of inflammatory cytokines—such as IL-6, TNF-α—and adipokines like leptin and resistin, which perpetuate systemic inflammation and may directly impact joint tissues¹.
These molecules are now recognised as key drivers of chronic, low-grade inflammation seen in metabolic syndrome, and are increasingly implicated in the development and progression of osteoarthritis (OA)².
Observational Evidence: VAT and Joint Pain
Several population-based and imaging studies suggest that central obesity, especially visceral fat, correlates more strongly with joint pain than body mass index (BMI) alone.
- A systematic review and meta-analysis by Walsh et al. showed that increased body fat is positively associated with musculoskeletal pain across multiple sites, including back, knees, and feet³.
- A 2025 study using the Visceral Adiposity Index (VAI) demonstrated a strong association between higher VAI quartiles and osteoarthritis prevalence in adults over 60⁴.
- In juvenile idiopathic arthritis (JIA), higher VAT levels (measured by imaging) were associated with significantly elevated serum IL-6 and resistin, suggesting metabolically active VAT contributes to systemic inflammation in inflammatory arthritis⁵.
These studies collectively indicate that VAT—not just overall adiposity—is closely linked with both inflammatory signalling and symptomatic joint disease.
Mechanistic Studies: How VAT Could Drive Joint Pain
Inflammatory mediators secreted by VAT—such as IL-6, TNF-α, and leptin—have been shown to:
- Activate synovial macrophages and fibroblasts, promoting synovial inflammation.
- Stimulate production of matrix metalloproteinases (MMPs), leading to cartilage degradation.
- Sensitise nociceptors in joint tissues, enhancing pain perception.
Animal models further support this pathway. In high-fat diet–induced obesity models, mice developed accelerated OA with increased cartilage erosion and synovial thickening. Importantly, these changes correlated with increases in circulating IL-6 and TNF-α⁶.
Furthermore, adipokines like leptin can act directly on cartilage and bone cells, altering metabolism and promoting catabolic activity in joint tissues⁷.
Clinical Interventions: Does Weight Loss Reduce Joint Pain?
Knee Osteoarthritis and Weight Loss
Most clinical evidence comes from studies on knee osteoarthritis, where both mechanical load and inflammatory processes play a role.
- The STEP-9 trial, published in NEJM in 2024, examined semaglutide 2.4 mg weekly in patients with obesity and knee OA. It showed significant weight loss (~13.7%) and a clinically meaningful reduction in knee pain compared with placebo⁸.
- Physical function and patient-reported outcomes also improved, suggesting that the benefits extended beyond weight alone⁸.
- Previous studies have shown that 10% weight loss can lead to 50% improvement in OA symptoms, but did not account for the role of VAT or inflammation specifically⁹.
Systemic Inflammation Responds to VAT Loss
A 2020 study by McLeod et al. examined dietary weight loss and exercise interventions. They found that VAT loss, more than total weight loss, correlated with reductions in CRP, an acute-phase marker of systemic inflammation¹⁰.
Another meta-analysis by Bulmer et al. (2025) showed reductions in IL-6 and TNF-α in some—but not all—weight loss studies, pointing to variability in individual responses and fat loss localisation¹¹.
These findings reinforce the idea that loss of VAT, rather than total weight, is critical in reducing systemic inflammatory burden.
GLP-1 Receptor Agonists: A Unique Opportunity to Study VAT Effects
GLP-1 receptor agonists (GLP-1 RAs) such as semaglutide and liraglutide offer a new tool in obesity management. Beyond weight loss, they target visceral fat preferentially, reduce appetite, and may have direct anti-inflammatory effects.
STEP-9: Semaglutide and Knee Pain
- Participants had significant reductions in body weight and improved WOMAC pain scores compared with placebo⁸.
- Importantly, changes in pain scores were not proportional to BMI reduction, suggesting non-mechanical pathways, potentially mediated by inflammation⁸.
Potential Anti-inflammatory Properties
Experimental models indicate that GLP-1 RAs reduce macrophage infiltration in VAT, suppress IL-6 and TNF-α production, and improve insulin sensitivity. This systemic anti-inflammatory shift could theoretically reduce joint inflammation, particularly in OA⁶.
A 2022 review by Meurot et al. proposed that GLP-1 may modulate synovial cell activation, suppressing catabolic cytokine release in joints¹².
What About Joints Beyond the Knee?
While most trials focus on knee OA, patients in real-world settings report improvements in:
- Hip and back pain, suggesting a broader anti-inflammatory or neuromodulatory benefit.
- Small joint pain (hands, fingers), where mechanical load reduction is minimal, pointing to systemic mechanisms.
However, randomised data for these joints is lacking, and observational findings need confirmation through dedicated trials.
How Strong is the Inflammation Hypothesis?
We propose the following model:
- Visceral adipose expansion → hypoxia, macrophage recruitment, IL-6 and TNF-α secretion.
- Systemic inflammation → synovitis, pain sensitisation, cartilage degradation.
- Weight loss, especially VAT reduction, leads to:
- Lower IL-6, TNF-α, leptin levels.
- Decreased nociceptor sensitisation.
- Improved joint function.
The following evidence supports this:
| Supported Findings | Areas Needing Clarification |
|---|
| VAT correlates with OA and joint pain⁴⁵ | Lack of RCTs in non-knee joints |
| GLP-1 RA therapy reduces pain independent of weight loss 8,12 | Contribution of specific cytokine changes needs better quantification |
| VAT loss correlates with lower CRP¹⁰ | Less consistent effects seen with IL-6, TNF-α in long-term studies¹¹ |
| Animal models show VAT inflammation worsens OA⁶ | Human joint imaging studies linked to VAT loss are lacking |
Future Research Directions
To better validate and apply this hypothesis, the following are needed:
- Mediation trials assessing whether changes in VAT volume and cytokine levels correlate with pain and function.
- Joint-specific studies beyond knees (e.g. hips, spine, fingers).
- Biomarker-guided trials, selecting patients with high VATI and elevated IL-6/TNF-α.
- Comparative studies of GLP-1 RAs vs. dietary/surgical weight loss to tease apart mechanisms.
- Long-term follow-up to assess joint structural outcomes (e.g. cartilage loss, joint replacement rates).
Clinical Takeaways
- For overweight and obese patients with joint pain, VAT is a valid therapeutic target.
- Interventions that preferentially reduce VAT, such as GLP-1 receptor agonists, may offer benefits beyond weight loss.
- Inflammation reduction, via lowering cytokine and adipokine release from VAT, may explain pain relief in joints that are not primarily weight-bearing.
- CRP and IL-6 could serve as biomarkers for VAT-driven joint pain in future clinical practice.
Conclusion
Weight loss remains one of the most effective non-invasive treatments for joint pain. But it’s time to move beyond the mechanical load hypothesis.
Emerging evidence shows that visceral fat is a pro-inflammatory endocrine organ, and that its reduction through GLP-1 therapy or caloric restriction leads to decreased cytokine production and systemic inflammation. This may explain improvements in joint symptoms—even in non-load-bearing joints like the fingers and spine.
While more mechanistic and interventional studies are needed, targeting VAT to reduce joint pain is a promising and actionable concept for both clinicians and patients.
References
- Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol. 2004;15(11):2792–2800.
- Zatterale F, Longo M, Naderi J, et al. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front Physiol. 2020;10:1607.
- Walsh TP, et al. Association between body fat and musculoskeletal pain: a systematic review and meta-analysis. Pain. 2018;159(4):610–618.
- Huang H, et al. Visceral Adiposity Index and Risk of Osteoarthritis: A Cross-Sectional Study. Front Nutr. 2025;8:1526377.
- Risum K, et al. Visceral adipose tissue is related to IL-6 and resistin in juvenile idiopathic arthritis. Rheumatol Int. 2025;45(1):88–94.
- Meurot C, et al. Targeting the GLP-1/GLP-1R axis to treat osteoarthritis. Int J Mol Sci. 2022;23(4):2213.
- Conde J, Scotece M, Gómez R, et al. Adipokines: biofactors from white adipose tissue. Implications for rheumatic diseases. Cell Biochem Funct. 2011;29(7):471–479.
- Bliddal H, Bays H, Czernichow S, et al. Once-Weekly Semaglutide in Persons with Obesity and Knee Osteoarthritis. N Engl J Med. 2024;390(12):1072–1082.
- Messier SP, et al. Intentional weight loss reduces knee osteoarthritis pain. Arthritis Rheum. 2013;65(5):1299–1305.
- McLeod A, et al. Impact of physical activity and VAT loss on inflammation: a meta-analysis. Obesity (Silver Spring). 2020;28(10):1780–1790.
- Bulmer C, et al. Effects of dietary weight loss on systemic inflammation: A meta-analysis. Obes Rev. 2025;26(3):e13910.
- Meurot C, et al. GLP-1 receptor agonists in OA: beyond glycaemic control. Int J Mol Sci. 2022;23(4):2213.
Other related articles
- Anthropometrics vs BMI: Why Waist Measures Outperform BMI in Cardiovascular Risk Assessment
- “Why Am I Out of Breath?” — The Hidden Link Between Belly Fat and Breathlessness
- Why everyone is talking about VAT
- How to Lose Visceral Adipose Tissue (VAT) and Improve Metabolic Health: A Guide to Sustainable Weight Loss
- The Expanding Waistline in Men: Spare Tyre, Killer Visceral Fat, or Just Flabby Abdominal muscles?
- From Genes to Greens: How DNA Shapes Your Nutritional Needs
- The 8-Month Metabolic Reset: A New Approach to Reversing Visceral Fat, Improving Blood Pressure and Blood Glucose